Schizophrenic Brain Cells Created in Lab
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
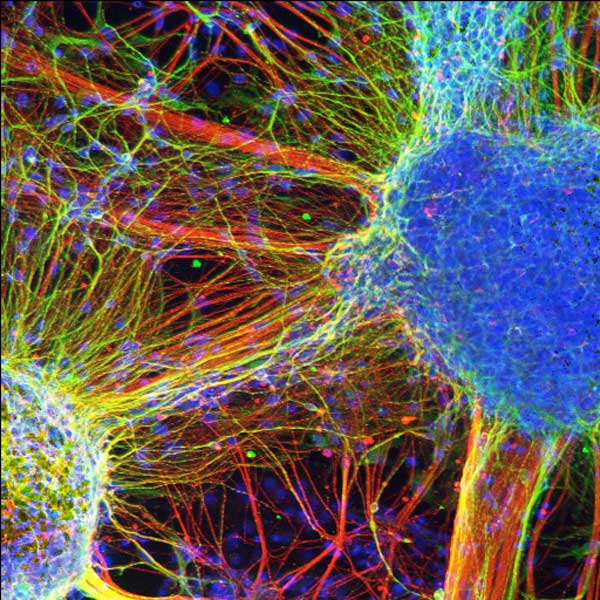
Skin cells taken from four individuals with schizophrenia have been turned into brain cells, or neurons, and grown in lab dishes, the first time a complex mental disorder has been examined using living brain cells.
The lab-grown neurons showed fewer connections between each other than was found in healthy brain cells, the researchers said.
The research not only will assist scientists in understanding the causes of a mental disease that plagues about 1 percent of the world's population (and about 3 million people in the United States), but also takes a step toward personalized medicine for those afflicted.
"What's so exciting about this approach is that we can examine patient-derived neurons that are perhaps equivalent to a particular patient's own neural cells," said researcher Gong Chen, an associate professor of biology at Penn State. The method would also allow researchers to test which drugs might work best for a particular patient without that person having to try it out first, Chen added. [Image of schizophrenic brain cells]
"The patient can be his or her own guinea pig for the design of his or her own treatment, without having to be experimented on directly," Chen said.
The research is detailed in the April 13 advance online issue of the journal Nature.
Cell smarts
Get the world’s most fascinating discoveries delivered straight to your inbox.
Challenges in studying psychiatric disorders such as schizophrenia include limited access to human brain cells as well as difficulty in teasing out genetic versus environmental influences on the disease, the researchers said.
"Nobody knows how much the environment contributes to the disease," said study researcher Kristen Brennand, a postdoctoral researcher at Salk. "By growing neurons in a dish, we can take the environment out of the equation and start focusing on the underlying biological problems." [Brain Cells in Lab Dish Keep Time]
And so the team, which also included Fred Gage, a professor in the Salk's Laboratory of Genetics, started from scratch in a way, turning the clock back on skin cells taken from four schizophrenia patients with a hereditary history of the disease. They programmed these cells to become unspecialized or undifferentiated stem cells called induced pluripotent stem cells. In this way, they avoided removing participants' neurons.
"A pluripotent stem cell is a kind of blank slate," Chen said. "During development, such stem cells differentiate into many diverse, specialized cell types, such as a muscle cell, a brain cell or a blood cell."
The team then directed the stem cells to become brain cells and compared the resulting neurons with those created from the induced pluripotent stem cells of healthy individuals.
Underpinnings of a disease
"Nobody knows how much the environment contributes to the disease," said Brennand. "By growing neurons in a dish, we can take the environment out of the equation and start focusing on the underlying biological problems."
And indeed they found some. Brennand treated the lab neurons with a modified rabies virus, which is known to travel along connections between brain cells. This tracer showed the schizophrenic neurons connected less frequently with each other and had fewer projections growing out from their cell bodies.
Genetic analysis also showed nearly 600 genes whose activity was off-kilter in these neurons, with 25 percent of these genes being linked to schizophrenia in past research.
The team tested the ability of five antipsychotic drugs — clozapine, loxapine, olanzapine, risperidone and thioridazine — to improve neuronal connectivity in the schizophrenia brain cells. Only loxapine significantly increased brain-cell connections from all schizophrenia patients, the researchers write.
At the end of the day, the results may help to counter social stigma often attached to mental disorders. "Many people believed that if affected individuals just worked through their problems, they could overcome them," Gage said. "But we are showing real biological dysfunctions in neurons that are independent of the environment."
You can follow LiveScience managing editor Jeanna Bryner on Twitter @jeannabryner.
Jeanna Bryner is managing editor of Scientific American. Previously she was editor in chief of Live Science and, prior to that, an editor at Scholastic's Science World magazine. Bryner has an English degree from Salisbury University, a master's degree in biogeochemistry and environmental sciences from the University of Maryland and a graduate science journalism degree from New York University. She has worked as a biologist in Florida, where she monitored wetlands and did field surveys for endangered species, including the gorgeous Florida Scrub Jay. She also received an ocean sciences journalism fellowship from the Woods Hole Oceanographic Institution. She is a firm believer that science is for everyone and that just about everything can be viewed through the lens of science.
 Live Science Plus
Live Science Plus










