Watch Ring-Shaped Molecule Unravel in Record-Fast Movie
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
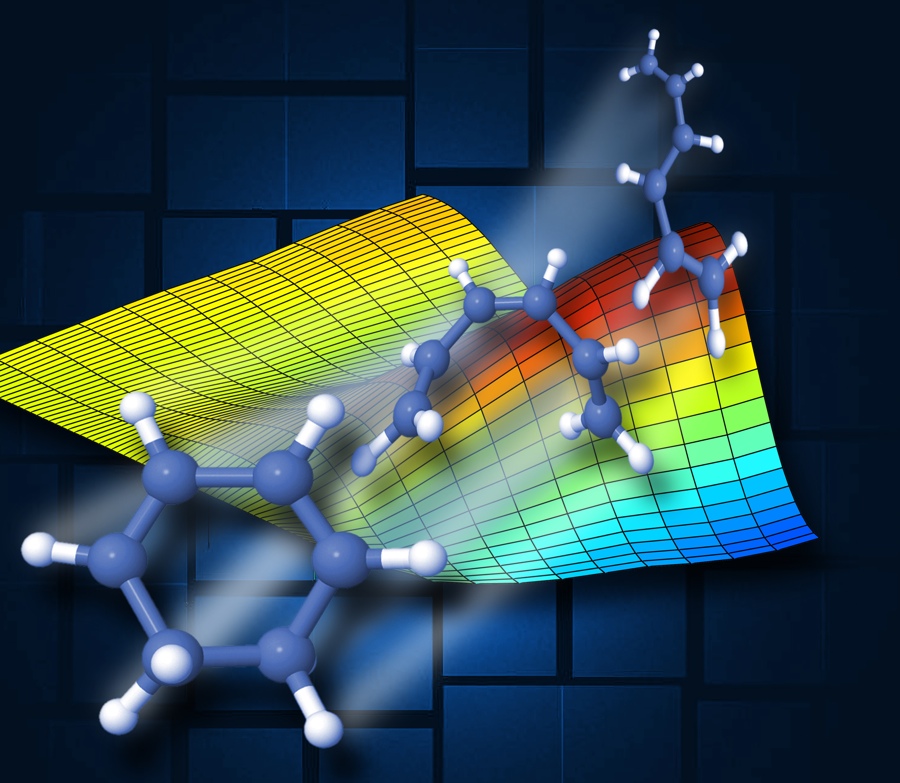
A molecule has become the world's smallest movie star.
For the first time, scientists have observed a chemical reaction as it was happening at the molecular level, at speeds that previously were too fast to see. The experiment could lead to insights about how complex molecules behave and why they take the shapes they do.
At the SLAC National Accelerator Laboratory, a team of researchers used two laser beams — one in the ultraviolet and another in the X-ray wavelengths — to get a picture of a chemical called 1,3-cyclohexadiene (CHD) as it morphed into another form called 1,3,5-hexatriene. They captured images of the reaction on a scale of femtoseconds, or millionths of a billionth of a second. [Watch the Ultra-Fast Molecular Movie]
"We kind of know what CHD looks like," Michael Minitti, lead author of the new study and a staff scientist at SLAC told Live Science. "The issue was the steps between one form and another."
Such reactions are called electrocyclic, and they show up in a lot of different places — for example, it's one of the ways animals synthesize vitamin D from sunlight. Although they're common, electrocyclic reactions aren't so well understood. A big question for physical chemists has been what happens to a molecule like CHD after it gets hit by the UV light but before it turns into 1,3,5-hexatriene.
To make their movie, the researchers first put a gaseous form of the CHD into a chamber at very low pressure. Then, they fired the ultraviolet laser at it, breaking one of the carbon bonds. The next step was to use an X-ray laser to zap the molecule. The X-ray laser flashes lasted only a few femtoseconds, as the whole reaction from CHD to hexatriene takes less than 200 femtoseconds to complete.
The X-rays scattered off of the molecules, and by looking at a pattern of light and dark on a detector, the researchers could read the shape of the molecule. Firing the X-ray laser repeatedly over a tiny fraction of a second showed how the shape changed over time.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The technique is similar to X-ray diffraction used when investigating the structure of DNA or crystals. (In fact, the structure of DNA was discovered in just this way in the 1950s.) There are crucial differences, though: X-ray diffraction doesn't measure anything over time, so the resulting picture is static; the X-rays in this new experiment were generated by a laser; and CHD is a gas, unlike the DNA molecule. "Gas molecules don't have a structure," Minitti said. "It looks like someone sneezed on the detector."
When chemists can see the way the shape changes, it tells them how such chemicals transform in a specific way that wasn't known before. Molecules tend to go to states of minimum energy, just as a ball rolling between two hills will tend to fall to the bottom and stay there. Regions of high and low potential energy surround the molecule, and when that molecule changes shape, its atoms will tend to stay in the low-energy regions. That means the shapes are specific, and knowing what they are offers insight into the processes that create the final forms.
While the research team was able to see the CHD change, the resolution in time —corresponding to the number of "frames" in an ordinary film — wasn't quite high enough to see every step, Minitti said. Each "frame" was about 25 femtoseconds, so there would be about eight in the animation. In the next experiment, scheduled for January 2016, he hopes to get a better picture of the changes with smaller intervals. Even so, the new experiment shows that such molecular moviemaking is possible.
The study is detailed in the June 22 issue of the journal Physical Review Letters.
Follow us@livescience, Facebook & Google+. Original article on Live Science.

 Live Science Plus
Live Science Plus





