Nature's Antifreeze May Hold Key to Tissue Preservation
This Research in Action article was provided to LiveScience in partnership with the National Science Foundation.
Ever wondered how animals and plants can survive in sub-zero temperatures?
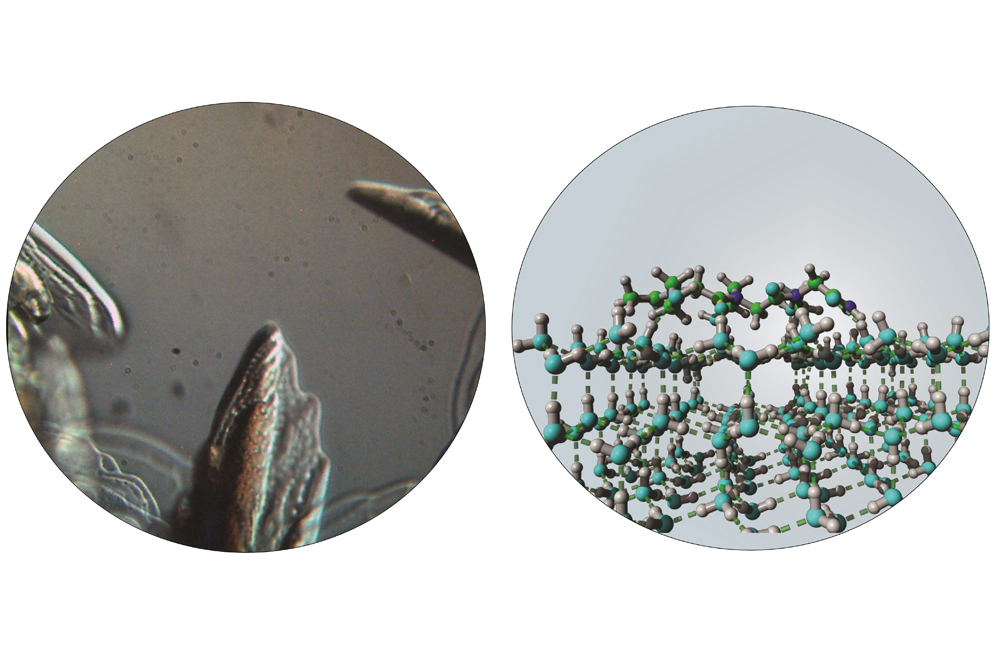
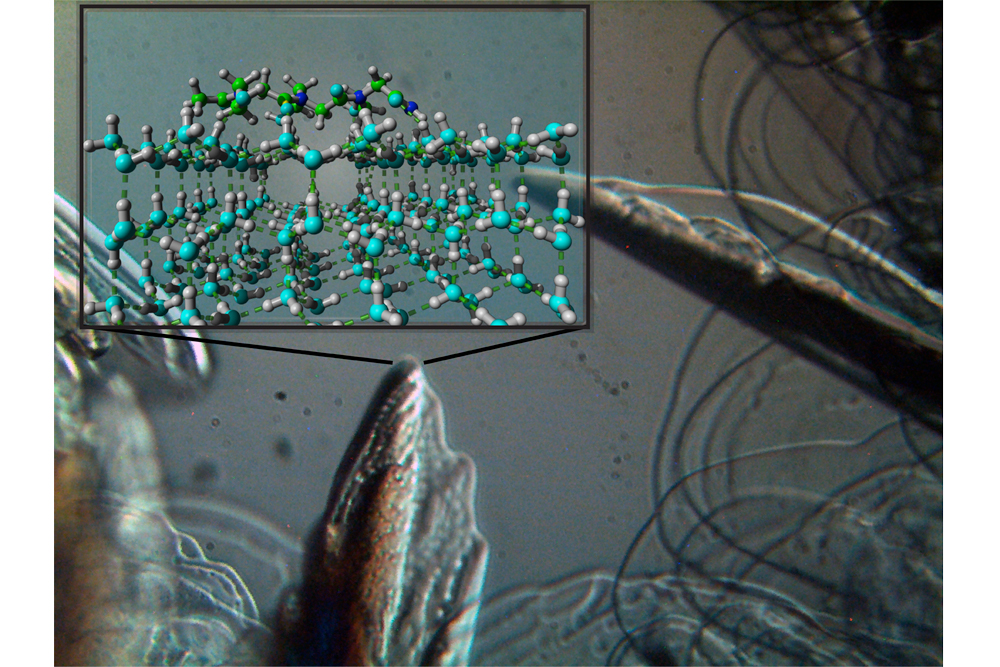
Natural compounds in these organisms can interact with the surfaces of ice crystals to slow freezing. With Nature as a source of inspiration, chemists at New York University have discovered synthetic molecules that can exhibit a similar ‘antifreeze’ effect.
Using microscopy and X-ray diffraction to observe the behavior of ice crystals, the scientists discovered that those peptide-mimetic compounds — aka “peptoids” — inhibit the growth of ice crystals and alter their melting temperature.
The antifreeze effects exhibited by those molecules are highly dependent on the details of their chemical structure. The antifreeze effects exerted by the new synthetic molecules may prove capable of freezing biological samples without damage, allowing long-term preservation of human cells or tissues.
Read more in the NYU announcement here.
Editor's Note: Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the National Science Foundation. See the Research in Action archive.
Get the world’s most fascinating discoveries delivered straight to your inbox.