Deaths Prompt CPSC, FDA warning on Infant Sleep Positioners

The U.S. Consumer Product Safety Commission (CPSC) and the U.S. Food and Drug Administration (FDA) today warned consumers to stop using infant sleep positioners. Over the past 13 years, CPSC and the FDA have received 12 reports of infants between the ages of 1 month and 4 four months who died when they suffocated in sleep positioners or became trapped and suffocated between a sleep positioner and the side of a crib or bassinet.
Most of the infants suffocated after rolling from a side to stomach position. In addition to the reported deaths, CPSC has received dozens of reports of infants who were placed on their backs or sides in sleep positioners, only to be found later in potentially hazardous positions within or next to the sleep positioners.
The deaths and dangerous situations resulting from the use of infant sleep positioners are a serious concern to CPSC, said CPSC Chairman Inez Tenenbaum. We urge parents and caregivers to take our warning seriously and stop using these sleep positioners, so that children can have a safer sleep.
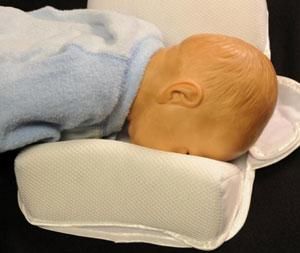
The two main types of infant sleep positioners are flat mats with side bolsters or inclined (wedge) mats with side bolsters.

Both types of sleep positioners typically claim to help keep infants on their backs and reduce the risk of Sudden Infant Death Syndrome (SIDS). The FDA has never cleared an infant sleep positioner to prevent or reduce the risk of SIDS. In addition, CPSC and the FDA are unaware of any scientific studies demonstrating that infant positioners prevent SIDS or are proven to prevent suffocation or other life-threatening harm.
To date, there is no scientifically sound evidence that infant sleep positioners prevent SIDS, said Dr. Joshua Sharfstein, FDA Principal Deputy Commissioner and a pediatrician. We want to make sure parents, health care professionals, and childcare providers understand the potential risk of suffocation and stop using infant sleep positioners.
Sleep positioners also typically claim to do one or all of the following: aid in food digestion to ease colic or the symptoms of gastroesophageal reflux disease (GERD); and prevent flat head syndrome (plagiocephaly). In light of the new safety data, FDA believes any benefit from using these devices to ease GERD or prevent plagiocephaly is outweighed by the risk of suffocation.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
CPSC and the FDA are warning parents and child care providers to:
The American Academy of Pediatrics does not support the use of any sleep positioner to prevent SIDS.
FDA has informed manufacturers of cleared devices of the agency's serious concern and has requested that they submit clinical data showing the benefits of their products outweigh the risk of suffocation or other serious harm.
Prompt reporting of adverse events can help the FDA and CPSC identify and better understand the risks associated with infant sleep positioners. If you have had a problem with an infant sleep positioner, the agencies encourage you to file a report through FDA's MedWatch program, at http://www.fda.gov/Safety/MedWatch/HowToReport/default.htm.
CPSC is interested in receiving incident or injury reports related to these products. Please visit https://www.cpsc.gov/cgibin/incident.aspx to file a report.
Most Popular


