Invasive crazy ants are being annihilated by murder fungus. Good.
The pathogen is driving populations of invading ants to extinction.

The days of invasive crazy ants — whose supercolonies can support millions upon millions of the fierce insects — may be numbered. That's because a deadly fungus that uses spring-loaded harpoonlike barbs to pierce the ants' gut cells is wiping out their colonies across the Southeastern United States.
That's not a bad thing. Tawny crazy ants (Nylanderia fulva), which are originally from South America, have over the past two decades become an increasingly problematic pest species and a threat to local wildlife in the U.S., by creating vast supercolonies.
Scientists with the University of Texas at Austin's (UTA) Brackenridge Field Laboratory recently identified a type of fungus that seemingly only targets tawny crazy ants, sparing native ant species and other arthropods. One ant colony infected with the fungus can spread the pathogen to others that are nearby, leading to the collapse of a supercolony and triggering the extinction of a crazy ant population within just a few years, the researchers reported in a new study.
In South America, tawny crazy ant nests are self-contained and the insects will battle ferociously with neighboring crazy ant colonies. But North America's invasive crazy ants follow a different strategy, in which new nests emerge out of an existing one — a process known as budding — and all the colonies' ants in a given area recognize each other as close relatives and move freely between nests, said Edward LeBrun, lead author of the new study and a research scientist in UTA's Department of Integrative Biology.
These nests "spread like bacterial plaque across a landscape," LeBrun told Live Science. "Every meter there's a nest, and that's over many square kilometers. How many ants are there? Many, many, many millions," LeBrun said.
Related: Murder hornets and monkey cannibals: 10 times nature freaked us out
Because crazy ants multiply quickly, they can swiftly become so numerous that they overwhelm local insects, arthropods, and small mammals and reptiles. They also swarm in human homes, multiplying by the thousands in basements, crawlspaces and walls, and even inside electronics, Live Science previously reported. But while forming supercolonies may have previously benefited crazy ants, living in a network of linked nests could prove to be their downfall by aiding the spread of a lethal pathogen, scientists reported March 28 in the journal Proceedings of the National Academy of Sciences.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
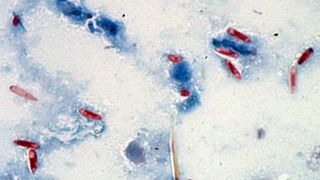
In 2015, LeBrun and his colleagues described a previously unknown microsporidian — a type of fungus — in tawny crazy ants that had been sent from Florida to their Texas laboratory. The ants had enlarged abdomens that were stuffed with white, fatty tissue, which happens when a microsporidia infection turns an ant into a spore factory, LeBrun explained. When the researchers checked Gulf Coast tawny crazy ant colonies, they found the fungus in local ants, too; they named the pathogen Myrmecomorba nylanderiae, taking the species name from the host ant.
M. nylanderiae spores are cylindrical capsules containing a tightly coiled filament tipped with a harpoonlike barb at one end. After a spore is swallowed by an ant, a chemical trigger in the insect's gut signals the spore to release the projectile within.
"If it happens to be close to the gut epithelium [a thin type of animal tissue], it'll puncture the cell wall of its host and then it injects the entire contents of the spore cell into the host cell," LeBrun said. The spore then hijacks the host cell's machinery to replicate itself, creating more spores and jumping into more cells, much as a virus replicates in a host, he explained.
Inoculating an infestation
For nine years, the researchers observed and sampled 15 infected and uninfected crazy ant colonies, discovering that all of the infected populations declined over time, and more than 60% of them disappeared completely within four to seven years of acquiring the pathogen. The scientists then tested the effects of the fungus by sending infected ants into uninfected crazy ant nests in Estero Llano Grande State Park in Weslaco, Texas. Within two years after introducing the pathogen, the park's previously "apocalyptic" crazy ant infestation had dwindled away into nothing.
The fungus did its lethal work by shortening the life spans of infected workers by about 23%, slashing a colony's workforce, LeBrun said. Workers would also transmit the infection to developing larvae, reducing the number of young that would develop into workers and ensuring that the next generation of workers would also be short-lived. Tawny crazy ant queens take a break from laying eggs during the winter, and don't resume egg laying until spring; in infected colonies, with every new egg-producing season there would be fewer new workers to care for the brood after the older workers died off. Over time, this would guarantee the colony's decline and eventual demise, according to the study.
Scientists don't yet know where the fungus came from — if it originated with crazy ants in South America, or if the ants first encountered it in the U.S. — but it doesn't seem to affect arthropods that are native to the Gulf Coast. This means that the fungus could be used to eradicate invading crazy ants and enable local species to safely return to the ecosystems where they once lived. But because the process of inoculating and tracking infection in a crazy ant colony is labor-intensive and technically challenging, it may be some time before this method is available as an off-the-shelf crazy ant solution for homeowners, Lebrun told Live Science.
"Its application is most likely going to be around areas of high conservation value or where there are endangered species, like state parks or national parks," he said.
Originally published on Live Science.

Mindy Weisberger is an editor at Scholastic and a former Live Science channel editor and senior writer. She has reported on general science, covering climate change, paleontology, biology, and space. Mindy studied film at Columbia University; prior to Live Science she produced, wrote and directed media for the American Museum of Natural History in New York City. Her videos about dinosaurs, astrophysics, biodiversity and evolution appear in museums and science centers worldwide, earning awards such as the CINE Golden Eagle and the Communicator Award of Excellence. Her writing has also appeared in Scientific American, The Washington Post and How It Works Magazine.
