Facts About Silver
Silver often plays second fiddle to another precious metal, gold, but this element has special properties that deserve a good look.
For example, of all the metals, pure silver is the best conductor of heat and electricity, according to the Jefferson National Linear Accelerator Laboratory. It's also the best reflector of visible light, which is why it is commonly used to make mirrors — though silver does tarnish and turn dark grey when exposed to air, requiring periodic polishing.
Pure silver is too soft for products like jewelry and tableware, so the family's finest forks and knives are most likely sterling silver, an alloy of 92.5 percent silver and 7.5 percent copper (though sometimes other metals are used). Silver is also used in some electronics and batteries. Because the metal has antimicrobial properties, nanoparticles of silver can be woven into clothing to prevent bacteria from building up on deposits of sweat and oils, according to the Royal Society of Chemistry (RSC).
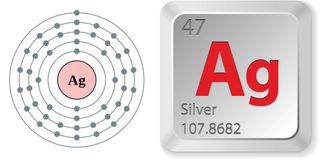
Just the facts
According to the Jefferson Lab, the properties of silver are:
- Atomic number (number of protons in the nucleus): 47
- Atomic symbol (on the Periodic Table of Elements): Ag
- Atomic weight (average mass of the atom): 107.8682
- Density: 10.501 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 1,763.2 degrees Fahrenheit (961.78 degrees Celsius)
- Boiling point: 3,924 F (2,162 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 66; 2 stable
- Most common isotopes: Ag-107 (51.839 percent natural abundance) and Ag-109 (48.161 percent natural abundance)
History of silver
Silver's history is long. The first evidence of silver mining dates back to 3000 B.C., in Turkey and Greece, according to the RSC. Ancient people even figured out how to refine silver. They heated the silver ore and blew air over it, a process called cupellation. The silver does not react to the air, but the base metals such as lead and copper oxidize and separate from the precious metal.
Silver forms in star explosions called supernovae, as does gold. A study published in September 2012 in the journal Astronomy and Astrophysics found that smaller stars that explodeproduce silver, while larger stars produce gold.

Silver really exploded on Earth, however, when Europeans landed on the New World in 1492. Spanish conquerors discovered that South America was home to rich veins of silver and silver ore, and they mined that wealth enthusiastically; according to the Silver Institute, an industry trade group, 85 percent of the silver produced worldwide came from Bolivia, Peru and Mexico between 1500 and 1800.
Silver played a big role in making early photography possible. Silver nitrate (silver combined with nitrogen and oxygen molecules) was used on photographic plates in the first, clunky cameras, according to the RSC, because it reacts to light by turning black — enabling photographers to capture an instant of light. Even with the rise of digital cameras, silver remains part of the traditional photographic process. As of 2003, the most recent year data is available, 1,920 metric tons of silver each year went to use for photographic purposes. Electrical and electronic uses were the second most-common single industrial use for silver, with 1,230 metric tons going into wires and gadgets in 2003. Jewelry, sterling silver and silver electroplated objects ran a distant third, using only 486 metric tons. Another 1,810 metric tons went to various other uses.
Who knew?
- Silver's atomic symbol is Ag, which seems to bear little relation to the name of the element. In fact, Ag is short for argentums, the Latin word for silver. The word "silver" is from the Anglo-Saxon word seolfor.
- The first huge silver strike in the United States was Nevada's Comstock Lode, first discovered in 1857 by two brothers who died before they could reap the benefits of their claim. According to Online Nevada, $305,779,612.48 of silver was pulled from the ground between 1859 and 1992.
- There's no doubt about it; silver is pretty, and humans have long thought so. In February 2014, archaeologists uncovered a trove of silver, including five hoop earrings, at a 3,200-year-old site in Israel.
- Silver has antimicrobial properties, but that doesn't make it a good option for home remedies. Homeopathy providers sometimes sell colloidal silver for a variety of health complaints, but drinking the stuff has a weird side effect: It turns the skin blue.
- Leave the good silver in a drawer between Christmases and it's likely to tarnish. So how do a museum's silver pieces stay so shiny? They're coated with transparent lacquers. Researchers are working to create nanometer-thick coatings that can replace the current hand-painted lacquers with something thinner, completely invisible and longer lasting.
Current research
Silver's antimicrobial properties have put this element in the doctor's bag of tricks; according to Wounds International, silver has been used to prevent the infection of injuries for hundreds of years. Silver doesn't kill microbes in its metallic form, in which it is unreactive. The metal works against bacteria only in ion form — it must lose an electron to become positively charged. The positively charged silver ion interferes with bacterial cell walls and disrupts other microbial processes.
Burn patients may use silver-antibiotic creams on their injuries, and some hospitals use silver-infused dressings for skin ulcers and other wound care. There are debates within the field, however, about the efficacy of these dressings, particularly after a 2010 review published in the Cochrane Database of Systemic Reviews found that they don't speed wound healing. A Wounds International working group of medical professionals, however, argued in 2012 that the dressings can be useful for localized infections.
Some manufacturers have taken silver's antimicrobial magic as a marketing opportunity, creating silver-infused textiles that purport to stop smelly bacteria from setting up shop in the fabric.
The problem, according to Bernd Nowack, a researcher at Empa, the Swiss Federal Laboratories for Materials Science and Technology, is that you don't know what you're getting in these silver-infused fabrics. In fact, manufacturers may not even know what they're making.
"They may add a compound but maybe during the manufacturing, all this dying and making of the fabric, they may transform some of the materials," Nowack told Live Science. In multiple studies, he and his colleagues have found that the forms of silver supposedly present in these high-tech textiles are rarely what are actually embedded in the fabric.
"They all contain a huge variety of different silver forms and sometimes the form that should have been in there was maybe 30 percent and the other 70 percent were other silver forms," Nowack said.
That matters, because some forms of silver don't interact as readily with bacteria as others. For example, fiber-embedded silver exposed to air literally tarnishes, combining with sulfur to make silver sulfide. Silver sulfide, Nowack said, is non-functional. It doesn't react to kill bacteria.
Ideally, a silver-infused fabric will use nanoparticles instead of conventional silver, because nanoparticles release at the right rate to keep a thin silver film on the fabric, preventing a bacterial foothold. Currently, though, Nowack said, there is no good way to point to a fabric and say for sure that the silver it contains is in nano-form. In fact, running a shirt with conventional silver threads through the washer will release more silver nanoparticles than running a nanosilver-infused shirt through the rinse cycle, Nowack and his colleagues reported in the journal ACS Nano in June 2014.
There are ways to improve the performance of silver-infused textiles, Nowack said, including embedding the silver directly into the fibers rather than giving them a surface treatment. Ultimately, though, silver may be too reactive to be a good fit for fabric. It does no good, after all, if your clothes tarnish like your silverware.
"As long as you have it exposed to air, you cannot inhibit this [tarnish], it can just happen," Nowack said. "It's something that so far hasn't really been considered."
Follow Live Science @livescience, Facebook & Google+.
Additional resources
- U.S. Geological Survey: Silver Statistics and Information
- The Silver Institute: The latest news and current facts and figures about silver
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
