
CVS-Brand Nasal Spray Recalled for Potential Bacterial Contamination
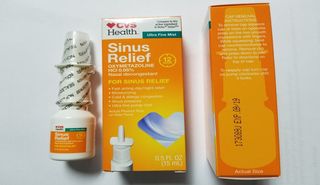
A CVS-brand nasal spray is being voluntarily recalled due to potential bacterial contamination, the U.S. Food and Drug Administration (FDA) announced today (Aug. 8).
The recall applies to Lot# 173089J of CVS Health 12 Hour Sinus Relief Nasal Mist, a nasal decongestant, according to the FDA statement.
The nasal spray is manufactured by a Florida company called Product Quest Manufacturing, which initiated the voluntary recall after discovering that the product was contaminated with a bacterium called Pseudomonas aeruginosa. [6 Superbugs to Watch Out For]
The symptoms of Pseudomonas infections depend on what part of the body becomes infected with the bacteria. For example, if the bacteria get into the lungs, a person can develop pneumonia, according to the Centers for Disease Control and Prevention (CDC). Pseudomonas bacteria can also cause ear, skin, eye and blood infections.
People who are hospitalized or those with a weakened immune system are most at risk for a Pseudomonas infection, the CDC says. In these groups of people, the infection can lead to severe illness and death.
Infections are treated with antibiotics, though they are becoming more difficult to treat as the bacteria develop resistance to the drugs, according to the CDC.
Repeated use of the recalled nasal spray could potentially lead to a buildup of the bacteria in a person's body, which could make them sick, the FDA said. In addition to people with weakened immune systems, people with cystic fibrosis are also at risk for life-threatening complications from this infection, the FDA said. (Cystic fibrosis is a disease that causes damage to the lungs, digestive system and other organs, according to the Mayo Clinic.)
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
To the best of Product Quest's knowledge, the company hasn't received any reports of adverse events related to the product that's being recalled, the FDA statement said.
People who have purchased the recalled product should stop using it immediately and return it to the place of purchase or throw it away. Anyone with questions regarding the product can contact Product Quest Manufacturing at 386-239-8787.
Originally published on Live Science.

Most Popular

