Comet Crashes Can Spawn the Ingredients of Life
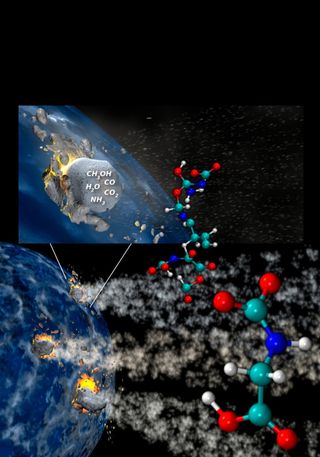
The explosive collisions of icy comets with planets and moons generated the vital building blocks of life, spreading these necessary ingredients throughout the solar system, researchers say.
"The important implication is that the complex precursors to life are widespread, thus increasing the chances of life evolving elsewhere," study co-author Mark Price, a space scientist at the University of Kent in England, told SPACE.com.
Comets are known to possess organic compounds. Scientists have long suggested that comets helped bring the ingredients of life to the early Earth. [7 Theories on the Origin of Life]
Astronomers have detected ammonia and other compounds in comets such as Halley's Comet that are the precursors of amino acids, the basic components of proteins. Indeed, the simplest amino acid, glycine, was recently discovered in samples of the Comet 81P/Wild-2 collected by NASA's Stardust spacecraft.
However, more complex amino acids are needed for life. Computer models from physical chemist Nir Goldman at the Lawrence Livermore National Laboratory in California suggested impacts could form complex amino acids, and Price and his colleagues set out to replicate these simulations, while astrobiologist Zita Martins at Imperial College in London and her colleagues helped look for any resultant amino acids.
"Impacts are ubiquitous in the solar system — we see impact craters on every solid surface in the solar system," Price said. "Due to gravity, we know these impacts must occur at very high velocities, kilometers per second. During such impacts, pressures and temperatures get very high, providing an environment that can induce chemical changes in target and projectile materials. One such change is that simple molecules can become more complicated ones."
In experiments, the researchers fired steel projectiles at speeds of up to 16,000 mph (25,200 km/h) at ice mixtures similar to ones found in comets. The targets could be difficult to work with — "a mix of carbon dioxide ice, ammonia and methanol gets extremely cold, minus 80 degrees Celsius (minus 112 degrees Fahrenheit), and handling the ices and containers meant using several layers of clean gloves, face masks and coveralls," Price said. "Even so, this still resulted in frostbitten fingers!"
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
The results included several amino acids, including L-alanine, an important component of proteins on Earth. Martins, Price, Goldman and their colleagues detailed their findings online Sunday (Sept. 15) in the journal Nature Geoscience.
Price cautioned, "We have not created life. Not even close. What we have done is demonstrate a process that takes molecules that were present at the time of the birth of the solar system and made them into molecules that are required for life. It's like taking simple LEGO bricks and sticking two together. You are a long way from building a house, but it is a start."
The researchers suggest that icy impacts — whether from icy comets against rocky planets or rocky or icy bodies against icy surfaces such as the moons of Jupiter and Saturn — could have manufactured complex organic molecules.
"As impacts occur everywhere we look, this implies that complicated molecules are also widespread throughout the solar system," Price said. "We have managed to generate a result that may increase the chance of life being present in an environment outside of the Earth, such as under the ice of Enceladus or Europa."
Future research can analyze what other compounds might form during such impacts — for instance, whether complex molecules can be altered into even more complex molecules.
This story was provided by SPACE.com, a sister site to LiveScience. Follow us @Spacedotcom, Facebook and Google+. Original article on SPACE.com.

Most Popular




